Optimal Microbial Sampling of Air Criteria
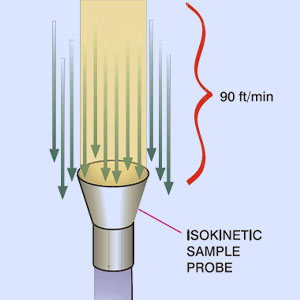
Microbial sampling of air in pharmaceutical manufacturing facilities is performed using several methods: surface sampling, passive aerosol (air) environmental sampling, and active aerosol (air) environmental sampling. Each of these methods produces a result based on the growth of microbial colonies on the surface of a collection media (typically agar), but the specific instruments and techniques vary between the methods. For all the sampling methods, it is important to be sure that the sample accurately reflects the environmental microbiological condition. This is often measured and discussed in terms of collection efficiency. The focus of this paper will mainly be on active air sampling, but there are sampling characteristics for each type of sampling that ensure the optimized collection efficiency.